
CSAP Management: Uwe P Tigör from Auxilius Pharma in Conversation with PharmaShots
Shots:
-
Chronic Stable Angina Pectoris (CSAP) affects over 11M patients in the US. Often considered as the first manifestation of an underlying coronary disease, there is a dearth of new-age innovative anti-anginal drugs
-
Today at PharmaShots we have Uwe P Tigör, Co-founder and Chief Medical Officer at Auxilius Pharma, shedding light on the positive results from Ph1a study evaluating AUX-001 for treating regular chronic stable angina pectoris (CSAP)
-
Auxilius Pharma currently focuses on a single area with two other pipeline candidates in development
Saurabh: Congratulations on the successful Ph1a (PK & safety) results of AUX-001! Would you mind shedding some light on the trial design?
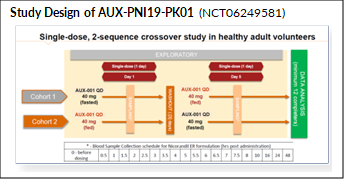
Uwe: Thank you and we are happy to share it (see enclosed study design image; for further reference see: https://classic.clinicaltrials.gov/ct2/show/NCT06249581]. Our first-in-human study with AUX-001 was a Phase 1a pharmacokinetic (PK) study designed with some core objectives in mind. Safety and tolerability were naturally right at the top. AUX-001’s reference product has been in clinical use in Europe for more than 2 decades and has an established record of being safe and tolerable. As a vasodilator, it does have a few well-defined – mostly transitory – issues typical for this class of medications. Discovering “no surprises” in this regard was very important for us so we can move on to other stages of clinical development. But clearly, this being a PK study we were also testing our innovative formulation to see if once-daily dosing is in reach for AUX-001.
Saurabh: Can you talk about the results of this Ph1a study being conducted on healthy volunteers?
Uwe: Chronic angina pectoris is a condition affecting almost exclusively adults making the use of adult healthy volunteers the obvious choice.

Amazingly, our capsule pushed the half-life time (T1/2) of the currently available twice (or in some countries three) times daily immediate release nicorandil from about 50min to over 9 hours. This would enable AUX-001 to be used once-daily for chronic angina, a condition marked by chest pain triggered by physical exercise in patients with underlying coronary disease, by giving it in the morning. We do, however, want patients to have the choice to take our medication either at bedtime or in the morning and will try to further optimize the drug release and extend the half-life time even more.
AUX-001 was safe and well tolerated and the extended-release formulation caused no severe adverse events at all. Almost all side effects were mild and consisted of the well-known issues nicorandil causes upon treatment initiation, mainly headaches. We were on the lookout for this, and our protocol called for the proactive, immediate treatment of headaches with simple IV acetaminophen. And that worked really well with no patients dropping out of the study or reporting their headaches to worsen to more severe levels.
Any vasodilating medication always gets scrutinized for temporarily lowering blood pressure at treatment initiation and we are happy to report that both systolic and diastolic levels of BP stayed well within normal ranges across our patients and returned to baseline within 24h.
Immediate-release Nicorandil can be given on an empty stomach or with a meal – so we looked into that as well with our once-daily formulation. We are a bit limited in our ability to go into details on the results due to submitted abstracts and IP concerns – but I hope this paints on overall picture.
Parts of our study results have been accepted for oral presentation at the annual meeting of the European Society of Cardiology, the 2nd largest global cardiovascular conference, and will be featured in the regular program (Abstr.88197, Session: 3110. Novel Drugs in Cardiovascular Disease). That shows just how much the interest level is rising on chronic angina R&D. We will present our topline results there on September 1st, 2024, at 2:30PM and hope it will allow physicians and investors to scrutinize the data in more detail.
Saurabh: What subsequent steps Auxilius Pharma intends to take to advance AUX-001 through further phases?
Uwe: Our next big step on the regulatory side is filing our innovative new drug application (IND) with the FDA later this year. We already had a pre-IND meeting with the FDA in April 2022 and discussed how to develop AUX-001 and under which regulatory pathway. Our compound will apply for approval under the 505(b)2 pathway thus taking advantage of a lot of clinical and pre-clinical work that has already been done with AUX-001’s reference product, immediate-release nicorandil. In terms of clinical development this means we will move swiftly from phase 1 – which consists mainly of a bioequivalence study of AUX-001 compared with its reference product - straight into Phase 3.
Saurabh: Can you enlighten us with insights into potential additional indications and patient populations that may benefit from the Extended Release of Nicorandil?
Uwe: Our initial indication for AUX-001 will be for treating regular chronic stable angina pectoris (CSAP). Traditional thinking connects chronic chest pain to plaque buildup in the large coronary arteries and their main branches. But the new horizon in chronic angina is the treatment of blood flow obstructions in the smaller branches, or microvascular sections of the coronary artery tree. Here, nicorandil is currently one of very few angina medications that appears to have a real impact on microvascular angina – and we plan to look into that with AUX-001 as well.
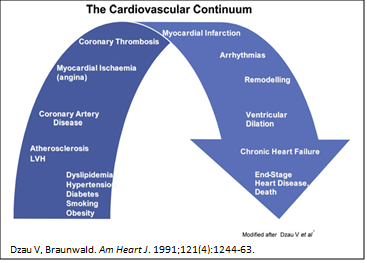
Nicorandil is a medication with a very interesting ability to affect aspects of coronary heart disease and its consequences along its trajectory. You have to think of ‘heart disease’– which sounds a bit generic – in its most common form in developed countries as a spectrum of conditions that start with cardiovascular risk factors, leads to the development of disease driving conditions such as high blood pressure and high cholesterol (hyperlipidemia) resulting eventually in atherosclerotic coronary disease with its typical plaques in the coronary artery tree of the heart. This disease, if not well controlled, will get worse causing symptoms such as chronic angina and eventually lead to heart attacks. If survived, that in turn can lead to the development of heart failure. Drs. Eugene Braunwald and Victor Dzau first described this cardiovascular disease continuum in their landmark paper in 1991 and this thinking has stood the test of time. Nicorandil via its unique dual mechanism of action has an effect on the heart’s vessels and muscle that we refer to as ischemic preconditioning which is thought to prepare the heart muscle to maintain functioning despite suboptimal blood oxygen supply conditions. This is partly how it affects chronic angina symptoms – but it has also been demonstrated to lower the risk or extent of future coronary events, such as heart attacks. Some studies even demonstrated that nicorandil improved heart failure caused by atherosclerotic coronary disease (i.e., ischemic cardiomyopathy), which in the context of the mentioned cardiovascular disease continuum would make a lot of sense.
Of course, any of these plans will only be possible with additional funding and partnering and does not affect our laser focus on timely approval of AUX-001 in its CSAP indication. We have been very lucky to attract investment throughout the past 4 years but especially 2023, when many small Biotech companies ran out of funding.
Saurabh: Is Auxilius Pharma looking for strategic partners for further development and commercialization?
Uwe: Yes, we are. With the successful conclusion of our first-in-human clinical trial we have opened our partnering window to explore this option – and we have already seen some positive responses from the several companies we have been put in touch into. We anticipate to partner up with a larger pharmaceutical player with a strong interest in heart disease before the start of Phase 3. As we will near the IND submission, we expect to retain a broker to ensure that all the interested parties have a chance to consider exciting this opportunity. Our initial focus is on the US market, however, we certainly eye also the EU and selected Asian countries, China in particular. We can imagine both a scenario where we choose one, global partner, but also a scenario where we end up working with different partners for major geographies.
Saurabh: How does Auxilius Pharma plan to utilize the drug's exclusivity globally?
Uwe: Thus far, we have successfully executed an international patent strategy and recently we have entered a national stage, wherein we prosecute patent application in major parts of the world, including Europe, China, India, and other parts of the Asia Pacific rim. We are working with one of the best pharmaceutical patent boutique firms in the US and I can disclose that we anticipate further strengthening our robust patent protection.
Saurabh: Can you provide an overview of the broader pipeline beyond AUX-001, including additional R&D projects or any future collaboration in various therapeutic areas?
Uwe: Since its inception in 2019, Auxilius has developed expertise in chronic angina which, with 11 million patients in the US alone, is one of the largest cardiovascular market segments, and our pipeline reflects that. We currently have 2 additional chronic angina compounds in development.
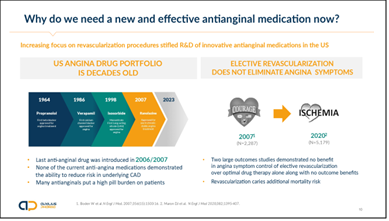
Why focus on this one area, you may ask? It presents a tremendous opportunity – both clinically as well as commercially – to improve treatment of chronic angina sufferers, whose numbers keep on rising. Yet, there has been a lack of developing anti-angina medications since the last launch here in the US in late 2006, which owes much to our belief that we could deal with chronic angina and its underlying disease companion coronary disease by putting stents into the coronary artery tree wherever there is an atherosclerotic plaque induced narrowing. Understandable in its approach, it took more than a decade worth of outcomes trial data to highlight that this approach does not work for chronic angina patients and that optimized medical therapy, the official term for drug treatment of chronic angina, is just as good as coronary stenting – and even coronary artery bypass grafting (CABG) – in most patients with less risk. Nevertheless, I can disclose that we are actively looking at other heart disease indications, particularly ones with significant overlap with CSAP.
The medications we have today in the US are, thus, a bit aged and many are still twice daily medications, serving a patient population that already take many medications to deal with the conditions that got them to chronic angina, including blood pressure, high blood lipids, often diabetes, to name a few. Developing new effective medications that treat angina with a simple once-daily application is therefore addressing a high unmet need and there is room for multiple new approaches. Auxilius is determined to address this challenge and AUX-002 and AUX-003 are representing this thinking.
Image Source: Canva
About the Author:

Uwe P Tigör
Uwe P Tigör, MD is Chief Medical Officer and one of the two cofounders of Auxilius Pharma in 2019. After his training in internal medicine in Germany and the US and a research fellowship in nuclear cardiology at The Mount Sinai Hospital in New York City that centered on diagnostic aspects of coronary artery disease and heart failure, he joined the life science industry and has worked in senior management positions at several New York City based medical marketing and consulting agencies servicing the pharmaceutical industry focusing particularly on regulatory and drug development consulting, as well as new medical product introduction and marketing.
Related Post: SEA Management: Donna Carstens from AstraZeneca in a Riveting Conversation with PharmaShots
Tags

Saurabh is a Senior Content Writer at PharmaShots. He is a voracious reader and follows the recent trends and innovations of life science companies diligently. His work at PharmaShots involves writing articles, editing content, and proofreading drafts. He has a knack for writing content that covers the Biotech, MedTech, Pharmaceutical, and Healthcare sectors.














